

AddH adds hydrogen atoms to molecules, as well as OXT atoms where missing from peptide C-termini. Chimera uses atom and residue names, or if these are not "standard," atomic coordinates, to determine connectivity and atom types; AddH then uses the atom types to determine the number of hydrogens to be added and their positions. The positions of pre-existing atoms are not changed, but any lone pairs and unidentifiable-element atoms are deleted. See also: FindHBond
 There are several ways to start
AddH, a tool in the Structure Editing category
(including using it via
Dock Prep).
AddH is also implemented as the command
addh.
There are several ways to start
AddH, a tool in the Structure Editing category
(including using it via
Dock Prep).
AddH is also implemented as the command
addh.
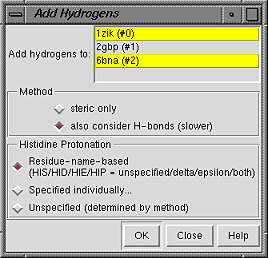
Models to which hydrogens should be added can be chosen from the list with the left mouse button. Ctrl-click toggles the status of an individual model. To choose a block of models without dragging, click on the first (or last) and then Shift-click on the last (or first) in the desired block.
Regardless of whether they are chosen for hydrogen addition, other models in the vicinity will affect hydrogen placement; if such interactions are not desired, the other models should be closed before hydrogens are added. Different submodels of the same model will not affect each other, however.
The Method for adding hydrogens can be:
If any atoms cannot be assigned a type, another dialog will appear. It is necessary to click on the line for each unassigned atom and then indicate its proper substituent geometry and number of substituents.
Added hydrogens are colored the element color (default white) if the attached atom is colored by element, otherwise the same as the attached atom.
The default VDW radii of carbon, nitrogen, oxygen, and sulfur atoms depend on whether hydrogen atoms are present. Therefore, the radii of some atoms will change when hydrogens are added.
AddH aims to generate protonation states reasonable at physiological pH. For example, hydrogens are not added to the phosphodiester moieties of DNA and RNA. By default, aspartic acid and glutamic acid sidechains are assumed to be negatively charged, while arginine and lysine sidechains are assumed to be positively charged (although other states can be attained). Two chemical moieties are treated as ambiguous at biological pH:
Residues at the ends of connected peptide chains are inspected to determine whether they are real termini, based on any SEQRES information in the input PDB file (or the mmCIF equivalent) and the presence or absence of additional chains with the same IDs. Real N-termini are assumed to be positively charged (+H3N–) and real C-termini are assumed to be negatively charged (–CO2–). If a C-terminal carboxylate is missing an oxygen (OXT), it will be added. End residues that are not real termini are terminated like other chain-internal residues, with N(H)– and –C(=O). The position of the N-end "amide" hydrogen in such cases is not fully determined by the positions of the existing atoms; AddH places this hydrogen to produce a φ angle equal to that of the subsequent residue.
Bond lengths for X-H (X = C/N/O/S) are taken from the Amber parm99 parameters:
| X | atom types | X-H bond length (Å) |
|---|---|---|
| sp3 carbon | C3 | 1.0900 |
| sp2 carbon | C2,Car | 1.0800 |
| sp carbon | C1 | 1.0560 |
| nitrogen | N3+,N3,Npl,Ng+ | 1.0100 |
| sp3 oxygen | O3 (except water) |
0.9600 |
| sp3 oxygen | O3 (water) |
0.9572 |
| sulfur | S3 | 1.3360 |
When a more intensive approach is desired, the program Reduce (developed by the Richardson Laboratory) is a good alternative. Reduce places hydrogens to optimize local H-bonding networks and avoid steric overlaps, while flipping certain sidechains 180 degrees as deemed appropriate to fulfill these criteria. Asparagine and glutamine sidechains may be flipped to switch their terminal N and O atoms, and the imidazole ring of histidine may be flipped to switch N and C identities. The protonation state of histidine is adjusted based on the local environment.
Reduce is available free at http://kinemage.biochem.duke.edu:
Asparagine and glutamine: using hydrogen atom contacts in the choice of side-chain amide orientation. Word JM, Lovell SC, Richardson JS, Richardson DC. J Mol Biol. 1999 Jan 29;285(4):1735-47.